ReSyn Biosciences
ReSyn Biosciences Ltd provides high-performance microsphere products for proteomics, molecular and cell biology applications.
Overview of our products for ReSyn BioSciences.











Magnetic reagents for life-sciences research, specializing in sample preparation for Mass Spectrometry and Proteomics.
ReSyn Biosciences developed a technology to produce highly porous microspheres which show a high number of benefits in comparison to solid microspheres. The South-African company is dedicated to enhancing the reproducibility and automation of mass spectrometry and bioseparation workflows by providing high-capacity and specificity magnetic microparticles. They are based on its proprietary microparticle technology platform. Please ask for your customized solution.
Technical advantages of ReSynBio's microspheres:
- High Capacity
- High Functional Group
- Density
- Multi-Point Covalent Attachment
- High Specificity
- HTS Compatible
Their MagReSyn® products are used widely and have proven their superiority in performance. The flexibility of the technology enables unique, customizable solutions that can be adapted easily into existing workflows.
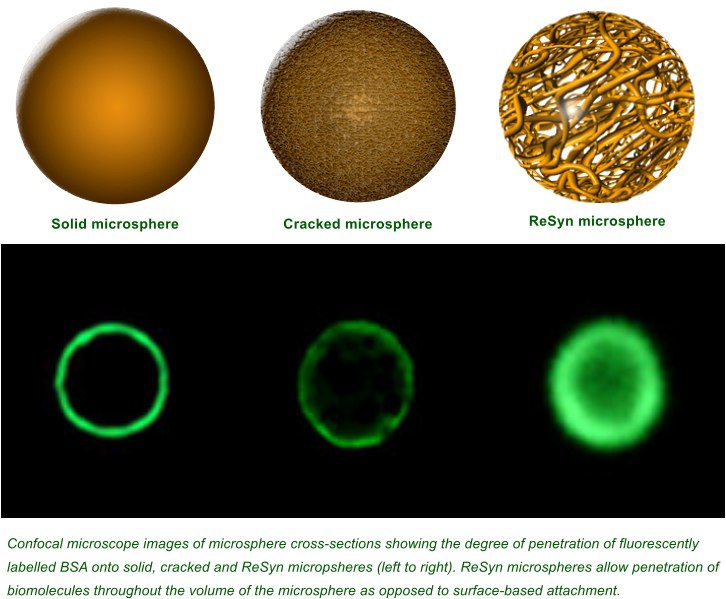
pic: ReSynBio - Confocal microscope images of microsphere cross-sections showing the degree of penetration of fluorescently labelled BSA onto solid, cracked and ReSyn microparticles (left to right). ReSyn microparticles allow penetration of biomolecules throughout the volume of the microparticle as opposed to surface-based attachment.
MagReSyn® TiO2
Protein phosphorylation plays a pivotal role in most cellular processes, with 30% of the proteome transiently phosphorylated. It is therefore a widely studied post-translational modification. However, the low-abundant nature of phosphopeptides, the low stoichiometry of the modification, and the physico-chemical properties of phosphorylated peptides make proteome-wide characterization of phosphorylation a significant challenge to proteomics researchers.
Consequently, technologies than can specifically enrich phosphopeptides, and are compatible with mass spectrometric analyses, are highly desirable. MagReSyn® TiO2 microparticles allow highly specific, reproducible enrichment of phosphopeptides from complex biological samples such as protein digests. Titanium dioxide enrichment shows selective affinity for phosphoserine (pSer), phosphothreonine (pThr) and phosphotyrosine (pTyr) residues. MagReSyn® TiO2 microparticles have been engineered to achieve the ultimate specificity, outperforming competitor products, with excellent phosphopeptide recovery.
TiO2 – functional microparticles for highly-specific phosphopeptide enrichment
MagReSyn® TiO2 microparticles may be used alone or in combination with MagReSyn® ZrO2, MagReSyn® Zr-IMAC and/or MagReSyn® Ti-IMAC microparticles to enrich diverse types of phosphopeptides for comprehensive phosphoproteomics analyses. Products for phosphopeptide enrichment are validated for the intended application using our stringent QC procedures.
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Bead size: ~5-10 µm
Formulation: 25 mg.ml-1 suspension in 20% ethanol
MagReSyn® ZrO2
The highly-specific capture of phosphorylated peptides from complex protein digests pose a significant challenge to proteomics researchers. Several technologies with varying degrees of specificity and affinity have been developed to enrich phosphopeptides. These different technologies can be used either alone or in combination to increase coverage of the phosphoproteome. MagReSyn® ZrO2 microparticles allow highly specific, reproducible enrichment of phosphopeptides from complex biological samples such as protein digests. MagReSyn® ZrO2 microparticles may be used alone or in combination with MagReSyn® TiO2, MagReSyn® Zr-IMAC and/or MagReSyn® Ti-IMAC microparticles to enrich diverse types of phosphopeptides for comprehensive phosphproteomics analyses. Products for phosphopeptide enrichment are validated for the intended application using our stringent QC procedures.
ZrO2 – functional microparticles for highly-specific phosphopeptide enrichment
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Bead size: ~5-10 µm average
Formulation: 50 mg.ml-1 suspension in 20% ethanol
MagReSyn® Ti-IMAC
Our classic and highly published research tool for phosphopeptide enrichment, consisting of phosphonate groups chelated with Ti4+.
Magnetic microparticles with chelated Ti4+ metal ions for highly-specific phosphopeptide enrichment
MagReSyn® Ti-IMAC has also been used in the development of new innovative sample preparation protocols.
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® Zr-IMAC
Our classic Zr-IMAC used for phosphopeptide enrichment. Now with optimized protocols enabling improved enrichment efficiency with high specificity (refer application note).
Magnetic microparticles with chelated Zr4+ metal ions for highly-specific phosphopeptide enrichment
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® HILIC
The automation of sample processing using magnetic beads reduces sample handling, and providing massively parallel sample processing, and improves reproducibility.
Multi-mode HILIC microparticles for sample clean-up pre-Mass Spectrometry
MagReSyn® HILIC provides a highly reproducible and fully automatable solution for parallel protein sample clean-up prior to MS analysis. The product is compatible with a range of common contaminants which interfere in the generation of high-quality data from MS analysis of samples.
Support: Proprietary polymer microspheres containing iron oxide (magnetite)
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® Hydroxyl
MagReSyn® Hydroxyl, consists of hydroxyl terminated magnetic beads. The hydrophilic surface and neutral functionality reduces non-specific interactions, increasing recovery of peptides in for instance applications requiring on-bead digestion.
Hydroxyl terminated beads
The ferromagnetic core and neutral functionality ensure fast and efficient magnetic separation for the transfer of beads, particularly suitable for automation of workflows on magnetic bead handling stations.
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® NTA & NTA Screening Kit
MagReSyn® NTA microparticles consist of nitrilotriacetic acid (NTA) residues with a strong metal-chelating property. MagReSyn® NTA is pre-chelated with nickel ions for affinity purification of histidine-tagged proteins. The product has been engineered for exceptional specificity, delivering highly pure target proteins.
For rapid affinity purification of 6x-histidine-tagged proteins
Since different types of His-tagged proteins may have varying affinities for a particular metal ion, we also offer a His-tagged protein purification screening kit (MagReSyn® NTA-KIT) containing MagReSyn® NTA chelated with 4 alternate divalent metal ions (Ni2+, Cu2+, Co2+, Zn2+) to identify the most suitable metal ion for optimal purification of your particular target protein. MagReSyn® NTA Copper, -Cobalt and -Zinc are currently available in 2 ml product formats.
Support: Proprietary polymer microspheres containing iron oxide (magnetite)
Binding capacity: > 1.0 mg.ml-1 (His-GFP)
Bead size: ~5-10 µm
Formulation: 25 mg.ml-1 suspension in 20% ethanol
MagReSyn® SAX
MagReSyn® SAX (strong anion exchange) is a magnetic polymeric microparticle support designed for capture, purification and recovery of biomolecules such as proteins/peptides, enzymes, antibodies by exploiting differences in net charge to reversibly adsorb oppositely-charged molecules.
For rapid protein fractionation of proteins by ion exchange & concentration and clean-up of proteins from dilute solutions pre-MS analysis
MagReSyn® SAX is ideal for fractionation of complex biological mixtures (e.g. serum, plasma, urine, CSF, cell lysates, culture supernatants etc.) prior to analytical methods such as 2-dimensional electrophoresis, HPLC or Mass Spectrometry. The microparticles have an exceptional capacity allowing for experimental miniaturization and elution in minimal volume.
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Binding capacity: > 20 mg.ml-1 (BSA) per ml
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® Amine
Amine (NH2) functional microparticles may be used to physically adsorb biomolecules through ionic interaction, or they can be chemically functionalized for covalent coupling of biomolecules using a variety of bi-functional chemical coupling agents. The most frequently used coupling agents include glutaraldehyde and 1,4-butanediol diglycidyl ether.
Primary Amine functional support for ligand adsorption or chemical modification
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Binding capacity: > 20 mg.ml-1 (BSA)
Bead size: ~5-10 µm
MagReSyn® Carboxyl
MagReSyn® Carboxyl is a magnetic polymeric microparticles support designed for covalent immobilization of primary amine-containing ligands through carbodiimide-succinimidyl ester activation and subsequent amide bond formation. Various strategies for the activation of carboxylate particles have been documented, but the most commonly used method for coupling proteins and other primary amine-containing ligands is through activation by EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide) in an aqueous reaction. This reaction can be either single-step or a two-step reaction requiring the addition of N-hydroxysuccinimide (NHS) or the more stable sulfo-NHS yielding NHS ester or sulfo NHS ester intermediates.
For covalent ligand immobilization via their primary amine residues
Please note that coupling of proteins through NHS-EDC activation can be tricky, requires fresh reagents, and is prone to interference from a range of routine buffers used in life-sciences. For simpler coupling of your ligand we would recommend using MagReSyn® Amine with glutaraldehyde or epoxide activation, or activation of MagReSyn® Carboxyl with commercial coupling reagents such as Anteobind™.
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Binding capacity: > 20 mg.ml-1 (BSA)
Bead size: ~5-10 µm
Formulation: 20 mg.ml-1 suspension in 20% ethanol
MagReSyn® Streptavidin
MagReSyn® Streptavidin microparticles contain covalently coupled streptavidin. The porous nature of the polymer technology allows for exceptional streptavidin capacity, translating into increased capacity for the immobilization/capture of biotinylated biomolecules. The high streptavidin load allows the use of as little as 10 µl beads, improving experimental miniaturization for high-throughput automated analysis. Molecular applications of MagReSyn® Streptavidin microparticles include, protein immobilization and purification; nucleic acid capture assays and preparation of single-stranded DNA templates for e.g. SELEX and pyrosequencing. The immobilization of biotinylated antibodies may subsequently be used in e.g. immunoassay.
High capacity for the capture of biotinylated molecules
Support: Proprietary polymer microparticles containing iron oxide (magnetite) with immobilized Streptavidin
Binding capacity (DNA): > 30 000 pmoles.ml-1 (Biotinylated oligo, 25-mer)
Binding capacity (Protein): > 3 mg.ml-1 (Biotinylated IgG)
Bead size: ~5-10 µm average
MagReSyn® Protein A and A-MAX
Ultra-capacity Protein A for the affinity purification of antibodies and immunoprecipitation
MagReSyn® Protein A microparticles contain covalently coupled Protein A for highly specific single-step purification of IgG to >95% purity from whole serum samples. Protein A is a ~56 kDa surface protein originally isolated from the cell wall of the bacterium Staphylococcus aureus and is widely used for highly specific purification of IgG antibodies and for immunoprecipitation experiments. Protein A has 5 immunoglobulin binding domains folded into a three-helix bundle and specifically binds the Fc region on the heavy chain of the IgG class of antibodies. More specifically, Protein A binds with high affinity to specific subclasses of human, rabbit, mouse, guinea pig, cat and pig IgG. It does not react with human IgG3, IgD or IgE, mouse IgM, IgA or IgE, or goat, koala and llama IgG. The high recombinant Protein A content of MagReSyn® ensures a high immunoglobulin capacity allowing for experimental miniaturization.
Support: Proprietary polymer microparticles containing iron oxide (magnetite) with immobilized Protein A
Binding capacity: > 2.4 mg.ml-1 (Rabbit IgG)
Bead size: ~5-10 µm
Formulation: 15 mg.ml-1 suspension in Tris buffer
MagReSyn® Protein A MAX microparticles have been specially engineered for custom applications where maximum antibody binding is desired. This includes microplate applications where miniaturization and high-throughput automated analysis are required.
Binding capacity: > 4.8 mg.ml-1 (Rabbit IgG)
Bead size: ~5-10 µm
Formulation: 15 mg.ml-1 suspension in Tris buffer
MagReSyn® Protein G
MagReSyn® Protein G is a proprietary magnetic polymeric microparticle support that provides a simple and convenient method for highly specific capture of various immunoglobulins from biological samples. Recombinant Streptococcal Protein G (lacking the albumin binding region for increased immunoglobulin specificity) is covalently linked to the magnetic microparticles reducing leaching during your experimentation, and potentially stabilizing the Protein G for applications in non-standard conditions. Protein G binds to most mammalian immunoglobulins with greater affinity than Protein A, including human IgG3 and rat IgG2a. It does not react with human IgM, IgD or IgA.
High capacity Protein G for affinity purification of antibodies and immunoprecipitation.
The high recombinant Protein G content of MagReSyn® ensures a high immunoglobulin capacity allowing for experimental miniaturization. MagReSyn® Protein G microparticles have been engineered to be highly specific, providing single-step purification of IgG to >95% from whole serum samples.
Support: Proprietary polymer microparticles containing iron oxide (magnetite) with immobilized Protein G
Binding capacity: > 2.4 mg.ml-1 (Rabbit IgG)
Bead size: ~5-10 µm
Formulation: 15 mg.ml-1 suspension in Tris buffer
MagReSyn® Trypsin
MagReSyn® Trypsin microparticles consist of covalently immobilized sequencing grade trypsin (TPCK treated to prevent chymotryptic activity) for protein digestion prior to mass spectrometry analysis. The porous high capacity MagReSyn® microparticle technology allows for high volumetric enzyme activity allowing for rapid digestion. The magnetically immobilized trypsin is stabilized against denaturation and retains functionality under denaturing conditions (including the presence of urea and SDS) with limited trypsin autolysis, allowing for new automated and miniaturized digestion workflows, improving digestion reproducibility.
For rapid, automated tryptic digestion of proteins for mass spectrometry sample preparation
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Digestion ratio: 20 µl (300 µg) microspheres per 50 µg protein sample
Bead size: ~5-10 µm
Formulation: 15 mg.ml-1 suspension in 50 mM acetic acid
MagReSyn® Chymotrypsin
MagReSyn® Chymotrypsin microparticles consist of covalently immobilized sequencing grade chymotrypsin (TLCK treated to prevent tryptic activity) for protein digestion prior to mass spectrometry analysis. The porous high capacity MagReSyn® microparticle technology allows for high volumetric enzyme activity allowing for rapid digestion. The magnetically immobilized chymotrypsin enables automated and miniaturized digestion workflows improving reproducibility. The enzyme cleaves at bulky hydrophobic residues thus providing complementary protein coverage as compared to magnetically immobilized trypsin digestion (see MagReSyn® Trypsin).
For rapid, automated chymotryptic digestion of proteins for mass spectrometry sample preparation
Support: Proprietary polymer microparticles containing iron oxide (magnetite)
Digestion ratio: 20 µl (200 µg) microparticles per 50 µg protein sample
Bead size: ~5-10 µm
Formulation: 10 mg.ml-1 suspension in 50 mM acetic acid
Facts and References about ReSynBio.
Ti-IMAC
Identification of ADP-Ribose Acceptor Sites on In Vitro Modified Proteins by Liquid Chromatography–Tandem Mass Spectrometry, Methods in Molecular Biology.
Leutert et al., 2017,
HILIC
Dysregulated healing responses in diabetic wounds occur in the early stages postinjury in Journal of Molecular Endocrinology
Amine
Spatial-proteomics reveals phospho-signaling dynamics at subcellular resolution.
Martinez-Val, A., Bekker-Jensen, D.B., Steigerwald, S. et al. Nat Commun 12, 7113 (2021). doi.org/10.1038/s41467-021-27398-y
Hydroxyl
Spatial-proteomics reveals phospho-signaling dynamics at subcellular resolution.
Martinez-Val, A., Bekker-Jensen, D.B., Steigerwald, S. et al., Nat Commun 12, 7113 (2021). doi.org/10.1038/s41467-021-27398-y
We are here for you.
Call us, write to us or make an appointment for a personal conversation
with us on site. Please do not hesitate to call, we love to be at
your service anytime.
PELOBIOTECH GmbH
Behringstrasse 10
82152 Germany
Phone +49 (0) 89 517 286 59 0
Mail info@pelobiotech.com

